During the COVID-19 crisis, we saw a spike in vaccine patent applications – but why does that matter? Because intellectual property rights (IPR) aren’t just legal protections; they’re the foundation of life-saving innovation. Without them, groundbreaking research wouldn’t have scaled into global solutions.
Curious about why IPRs matter to biotech and life sciences innovators, and how their use played out in the COVID-19 pandemic and beyond? Two IP patent attorney Sheena Linehan explains more.
In the realm of biotech and life sciences, the ‘Why’ of protecting intellectual property (IP) is deeply connected to the mission to advance human health. Safeguarding IP is essential for fostering innovation, allowing groundbreaking medical discoveries to be developed and scaled to address pressing health challenges.
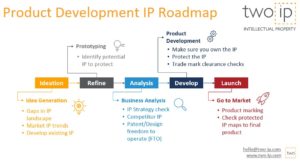
Why protecting Intellectual Property matters
The primary purpose of intellectual property rights (IPR) in this sector is to incentivise the development of new medical solutions. The process of creating vaccines, therapies, and medical technologies demands significant investment in research and development. By securing IP, organisations can protect their innovations from unauthorised use, allowing them to recoup investments and fund further research. This protection is crucial for translating scientific discoveries into accessible health solutions that benefit society at large.
Securing patents and other IP rights allows organizations to:
✅ Secure investment for R&D and clinical trials
✅ Form strategic partnerships for clinical development and large-scale manufacturing
✅ Enter new markets and foster global accessibility through licensing and collaborations
The COVID-19 pandemic highlighted a critical role for IP in public health. The University of Oxford developed a candidate COVID-19 vaccine ChAdOx1 and secured its IP. To ensure widespread access, they licensed this IP to AstraZeneca, which carried out clinical trials and franchised production to global partners, facilitating mass production and distribution. AstraZeneca notably partnered with the Serum Institute of India (SII), which alone manufactured over a billion doses. This collaboration was pivotal in delivering vaccines to low- and middle-income countries, demonstrating how strategic IP management can serve the public interest.
How IPR fuels ongoing innovation
The benefits of IP protection don’t end with one breakthrough. Following the success of the COVID-19 vaccine partnership, SII has pledged £50 million for a vaccines research facility at Oxford University, and has partnered with the University in development of the R21/Matrix-M malaria vaccine, which it will manufacture on a large scale.
Companies like Moderna and BioNTech, pioneers of mRNA vaccine technology, utilised their IP to rapidly develop COVID-19 vaccines, primarily for high-income countries. Post-pandemic, they are exploring the application of mRNA technology to cancer vaccines, showcasing how robust IP portfolios can drive ongoing innovation to address various health issues.
What are Intellectual Property Rights?
Intellectual Property Rights are legal protections granted to creators and innovators. In the biotech and life sciences sectors, these typically include:
- Patents: Protect new inventions, such as novel biologics and their medical uses, and medical devices, for a limited period of up to 20 years.
- Trade Secrets & Confidential Information: Protect proprietary formulae, research data, and manufacturing processes etc.
- Trademarks: Safeguard brand names and logos, ensuring market identity.
- Designs: Protect the unique appearance or design of a product, such as a medical device, for a limited period of up to 25 years.
These rights ensure that innovators can control the use of their creations, providing a framework to monetise their inventions and fund further research.
Impact of IPR on health
Owning IPRs significantly influences the ability of biotech and life sciences companies to address health challenges. The COVID-19 pandemic led to a substantial increase in vaccine patent applications, reflecting a surge in innovation aimed at combating the virus. This surge was driven by the need to develop effective vaccines rapidly, highlighting how IP protection can stimulate the creation of solutions to global health crises.
Moreover, strategic IP management facilitated collaborations that were essential for large-scale vaccine production and distribution. The partnership between AstraZeneca and SII, underpinned by IP licensing agreements, enabled the delivery of vaccines to numerous countries, exemplifying how IP can be leveraged to enhance global health outcomes.
In essence, for biotech and life sciences companies, the belief encapsulated in obtaining IPR is that innovation is a catalyst for improving health. Protecting IP not only rewards innovators but also ensures that their innovations can be developed, scaled, and distributed to address critical health challenges, ultimately serving the greater good.
Biotech Leaders – Protect your innovations
Biotech and life sciences companies must take action to protect their IP. Without it, groundbreaking research can be copied, funding becomes harder to secure, and the path to global impact becomes uncertain.
Our patent and trade mark attorneys can help you work out what you should be doing and then help you do it. To get in touch, click here or email us at hello@two-ip.com.
💡Found this information useful? Click here to sign up for our monthly Two Insights newsletter.
Sources:
£50m funding for Poonawalla Vaccines Research Building at Oxford University | University of Oxford
Merck and Moderna’s mRNA cancer vaccine aces its first efficacy trial | pharmaphorum